Published in: Michael A. Arbib (Ed.): The Handbook of Brain Theory and Neural Networks (second ed). MIT Press, 2002.
email: rob<at>retina.anatomy.upenn.edu
At the most basic level, the retina transduces spatial and temporal variations in light intensity and transmits them to the brain. However instead of directly coding intensity, the retina transforms visual signals in a multitude of ways to code properties of the visual world such as contrast, color, and motion. The purpose of this chapter is to develop a conceptual theory to explain why the retina codes visual signals and how the structure of the retina is related to its coding function.
The vertebrate retina reliably responds to light contrast as low as 1% (Shapley and Enroth-Cugell, 1984). Yet as the delicate visual signal is amplified in its passage through the retina, the biological limitations of neural processing add distortion and noise. The ease with which we see fine details in the presence of such biological limitations suggests that one function of retinal circuitry is to maintain the signal's quality by removing redundant signals to enhance signal quality (Laughlin, 1994). This hypothesis predicts that much of the retina's signal coding and structural detail is derived from the need to optimally amplify the signal and eliminate noise.
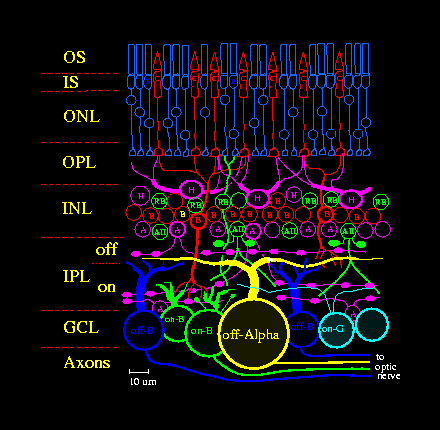
Neurons of each type are spaced in a regular array across the retina (see Figure 1), so the key to understanding retinal function is to identify the processing strategies of repeating functional circuits or modules (Sterling, 1997). To understand a retinal neuron's physiological function, investigators measure its "receptive field", the region in space and time over which it responds to light. Receptive fields of retinal neurons consist of a sensitive circular region in visual space called the "center", and a larger but weaker antagonistic region concentric with the center, called the "surround" (Rodieck, 1998), which are determined by intrinsic and presynaptic mechanisms. For example, a ganglion cell's receptive field shape and properties reflect its morphology and biophysical properties (Kandel et al., 2000), and also receptive field properties of its presynaptic bipolar and amacrine cells, which in turn originate to some extent in the receptive field properties of photoreceptors and horizontal cells (Dowling, 1987; Sterling, 1997; Rodieck 1998).
While receptive field analysis is a powerful method for studying the function of a neural circuit (see Rodieck, 1998; Shapley and Enroth-Cugell et al, 1984), the origin of a receptive field in a circuit that includes several layers of neurons is difficult to grasp. The difficulty is to separate the effects of cell morphology, synaptic connectivity, and membrane channels on the receptive field (e.g. see RETINAL DIRECTION SELECTIVITY). However, by computationally simulating these biophysical details based even on partial knowledge, it is possible to test specific hypotheses about neural circuit connectivity (Teeters and Arbib, 1991; Smith, 1995).
The outer segment (OS) of a vertebrate photoreceptor transduces light via a multi-step biochemical cascade (Rodieck, 1998; Kandel et al, 2000) into an electrical signal that is conducted through the photoreceptor's axon to its terminal in the OPL. In response to a flash of light, ion channels close, hyperpolarizing the photoreceptor proportionately over a limited range of stimulus intensity. The advantage of this coding function is that a photoreceptor responds well to low contrast signals common in the visual world. The disadvantage is that outside this limited range the photoreceptor responds poorly. At lower intensities, the photoreceptor's transduction gain (i.e. proportion of change in its output signal to a change in light) tends to be insufficient, and at higher intensities, the photoreceptor's response tends to saturate. To solve such saturation problems, the photoreceptor adjusts its gain in a process called "adaptation", which in some species can modulate transduction gain by up to 4 log units.
The two classes of photoreceptors, rods and cones (Rodieck, 1998), differ in that rods are sensitive to single photons and are bleached by daylight, but cones are less sensitive and can regenerate their pigment in daylight (photopic intensity range). At twilight (the mesopic intensity range), rods are coupled via gap junctions to neighboring cones, causing the rod signal to pass directly into cones where it is carried by the lower-gain cone pathway (Rodieck, 1998). At night (scotopic intensity range), a special "rod bipolar" pathway (RB in Figure 1) carries quantal "single photon" signals, removes dark noise, and adapts over an extra 3 log units of intensity (Sterling, 1997, Rodieck, 1998; Smith and van Rossum, 1998).
The OPL's high-pass filter is constructed by subtracting a "local average" from the cone. Horizontal cells, also coupled laterally by gap junctions, sum inputs from many cone terminals and provide negative feedback to each via a feedback synapse. The negative feedback mechanism in some cases is a GABA-ergic synapse (Dowling, 1987; Sterling, 1997), but has also recently been postulated to be a form of electrical feedback. The synaptic structure that performs this function, called a "triad", has both feedforward and feedback contacts so it is termed "reciprocal" (Dowling, 1987; Rodieck, 1998). This type of coding has been termed "predictive" (Laughlin, 1994) because the ideal signal to subtract would be a "local average" of signals from neighboring cones (Smith, 1995).
Bipolar cells respond as photoreceptors do with a voltage proportional to light intensity, but their response range is narrower and they adapt over a wider range of stimuli. Adaptation occurs at the dendritic tip from changes in gain at a second-messenger biochemical cascade, at the membrane by voltage-gated ion channels, or at the axon where gain of output synapses is regulated in several ways by feedback. Bipolar cells contact ganglion cells with glutamatergic ribbon synapses to allow high release rates and reduce noise. A bipolar cell may contact several ganglion cell types, each with a different characteristic number of synapses, which implies a specific coding of the bipolar signal (Sterling, 1997; Teeters and Arbib, 1991).
Amacrine cells are a diverse group in both morphology (Kolb et al, 1981; Rodieck, 1998) and neurochemistry (Masland, 1988). Many have a large (0.5-2 mm) but sparse dendritic field with very fine dendritic processes (0.2 um dia) that stretch between small swellings, called "varicosities", where synaptic connections are made (Kolb, et al, 1981; Dowling, 1987). Most amacrine cells contain voltage-gated Na+ channels and fire action potentials which allows them to transmit signals laterally over the extent of their dendritic field (Masland, 1988). Amacrine cells are generally either GABAergic (Kandel et al, 2000; Rodieck, 1998) or glycinergic, which implies that they perform subtractive or shunting control functions. Some, e.g. the cholinergic "starburst" amacrine, are involved in temporal processing, and respond transiently to light (Masland, 1988). Amacrine circuitry is thought to be responsible for accentuating the surround, directional selectivity in ganglion cells (see RETINAL DIRECTION SELECTIVITY), excitatory transient and peripheral effects, and several types of gain control (Shapley and Enroth-Cugell, 1984; Dowling, 1987; Rodieck, 1998).
Amacrine cells receive synaptic contacts from bipolar cells at a "dyad" where a bipolar ribbon synapse contacts two postsynaptic neurons, usually ganglion and amacrine cells (Rodieck, 1998). The similarity between the synaptic "dyad" in the IPL and the "triad" in the OPL is striking (Rodieck, 1998). Both contain synaptic "ribbons", and both include reciprocal feedback from a lateral neuron. The reason may be the identical problem of noise. The reciprocal feedback from an amacrine varicosity to its presynaptic bipolar cell can process the signal reducing its dynamic range before transmission to ganglion cells (Masland, 1988; Dowling, 1987; Rodieck, 1998).
Amacrine and bipolar cells, like many types of neuron in the brain, are widely coupled by gap junctions to their neighbors. Bipolar cell coupling, like cone coupling, correlates neighboring cells' signals to enhance synchronous vesicle release. Since many amacrines fire action potentials, one possibility is that gap junctions allow them to synchronize their firing. But their diversity emphasizes the complexity of retinal circuitry (Masland, 1988; Rodieck, 1998). The AII amacrine cell is small-field and carries rod signals from the rod bipolar to cone bipolars at night (Kolb et al., 1981, Rodieck, 1998). To grasp the function of the AII has been a special challenge because it is coupled by gap junctions to its AII and bipolar cell neighbors, and these two types of electrical coupling are controlled by independently-modulated second-messenger systems. The AII also contains voltage-gated Na+ channels and generates action-potential-like transients. These specialized biophysical properties elegantly solve a signal-processing challenge: in starlight, the AII collects single photon signals from an array of presynaptic rod bipolars, but synaptic noise is collected even when photons are rare. The AII's strategy, therefore, is to reduce noise by electrical coupling with neighbors, and to nonlinearly amplify the single-photon signal with voltage-gated channels (Smith and Vardi, 1995; Sterling, 1997) removing noise and reshaping the signal before passing it on.
To supply on- and off-ganglion cells with appropriate signals, the IPL is organized into on- and off-layers ("sublaminae"). Two bipolar cell subclasses, "on", and "off", respond oppositely to glutamate released by cones. Bipolar and amacrine cell types are divided roughly equally between the two layers, although some arborize in both. The on- and off-layers are in turn organized into specific sub-layers defined by microcircuits comprising bipolar, amacrine, and ganglion cells, each generating a specific spatio-temporal code (Sterling, 1997).
On-bipolar dendrites contain "metabotropic" receptors, which, when bound by glutamate released by a photoreceptor, signal a cytoplasmic second-messenger to turn off the synapse's ionic channels (Sterling, 1997; Dowling, 1987; Rodieck, 1998). Thus an on-bipolar depolarizes when the photoreceptor decreases its glutamate release (i.e. in response to light). An off-bipolar contains "ionotropic" glutamate receptors that directly open an ion channel and hyperpolarize to light. Each off-bipolar type contains glutamate receptors with different kinetic parameters which are the first step in generating a specific temporal code. Some bipolar cells code stimulus velocity, direction, or color (Haverkamp, et. al, 1999; Rodieck, 1998). These specializations increase signal fidelity which is an advantage for a visual signal that is destined to pass through a noisy channel to the brain.
Several reasons explain the diversity of retinal circuitry. By discarding part of the information it receives, a neuron specializes in coding specific properties of the signal, i.e. contrast, motion, bright, dark, or colored light flashes, etc. The exact details of the coding scheme are probably related to the ecological niche occupied by the organism. Rod signals because of their quantal nature are qualitatively different from cone signals so there is an advantage in a separate rod pathway. Such specialization in coding increases the signal/noise ratio and makes better use of the limited dynamic range of neurons, synapses, and the spike train in the ganglion cell axon (Laughlin, 1994). Specialization in coding also simplifies the task of brain circuitry in visual "segmentation" (Kandel et al, 2000), which implies a function for retinal circuit structure.
Receptive fields of many retinal neurons, and ganglion cells in particular, share important properties: their center-surround organization, high sensitivity to contrast, and wide-ranging adaptation. To the extent that each retinal circuit amplifies the signal, it adapts to reduce the signal's dynamic range, which implies that the retina's high sensitivity is achieved at the cost of complexity. For example, the net effect of the OPL circuit is to create for the photoreceptor a receptive field with a broad center region and a wide antagonistic surround (Sterling, 1997; Rodieck 1998; Kandel et. al., 2000) that adapts temporally and spatially. By removing information about absolute light intensity, the OPL circuit transmits what is left, i.e. information about contrast.
In turn, the IPL circuit removes more information about light intensity and contrast, shaping the signal in time to code transients, and accentuating the spatial center-surround receptive field in bipolar and amacrine cells (Rodieck, 1998; Dowling, 1987). This process further regulates the visual signal's gain to improve discrimination of low-contrast objects from noise and to prevent saturation at high contrast (Shapley and Enroth-Cugell, 1984). The result of these operations is that retinal receptive fields change with background intensity to maximize information transfer (Laughlin, 1994), and the consequence of this processing is the familiar center and surround of the ganglion cell. Thus it appears that circuits along the retinal pathway all contribute to the ganglion cell's receptive field properties for a similar reason: to prevent noise or saturation from degrading the signal (Laughlin, 1994).
The well-known antagonistic center-surround and adaptation properties of the ganglion cell receptive field, therefore, seem driven by the goal of preserving signal quality. To accomplish this, the circuitry of both OPL and IPL increase the receptive field's lateral extent. But the need for high visual acuity mandates that OPL and IPL circuits not extend laterally too far. Thus the retina is shaped to compensate for biological limitations by a compromise between spatial acuity and accuracy of coding.
Computational modeling promises to help find the answers (Teeters and Arbib, 1991; Fohlmeister and Miller, 1997; Smith, 1995; Smith and Vardi, 1995; Haverkamp et al, 1999). Once the basic signal flow and function in a retinal circuit have been established, simulations can help determine overall strategies, and with information theory can find what biological limitations are most serious to the circuit (Laughlin, 1994). The effect of noise on the retina's performance can be tested by simulating noise from all the sources in the signal pathway, and comparing the resulting signal/noise ratios as a measure of signal quality.
* = resource reference.
*Dowling, J.E., 1987, The Retina: An Approachable Part of the Brain, Cambridge, MA: Harvard University Press.
Fohlmeister, J.F., and Miller, R.F., 1997, Mechanisms by which cell geometry controls repetitive impulse firing in ganglion cells, J. Neurophysiol., 78: 1948-1964.
*Kandel, E.R., Schwartz, J. H., and T.M. Jessel, 2000, Principles of Neural Science, 4th Ed., New York, NY: McGraw-Hill.
Kolb, H., Nelson, R., and Mariani, A., 1981, Amacrine cells, bipolar cells, and ganglion cells of the cat retina: a Golgi study, Vision Res., 21:1081-1114.
Haverkamp, S., Moeckel, W., and Ammermuller, J., 1999, Different types of synapses with different spectral types of cones underlie color opponency in a bipolar cell of the turtle retina, Vis. Neurosci., 16:801-9, 1999.
*Laughlin, S.B., 1994, Matching coding, circuits, cells, and molecules to signals: General synaptic principles of retinal design in the fly's eye, Prog. in Retinal Eye Res., 13: 165-196.
*Masland R H., 1988, Amacrine cells, Trends Neurosci, 11:405-410.
Maturana, H.R., Lettvin, J.Y., and McCulloch, W.S., 1960. Anatomy and physiology of vision in the frog (rana pipiens), J. Gen. Physiol., 43:129-175.
*Rodieck, R.W., 1998, The First Steps in Seeing, Sunderland MA: Sinauer.
*Shapley, R.M., and Enroth-Cugell, C., 1984, Visual adaptation and retinal gain controls, Progr. in Retinal Res., 3:263-346.
Smith, R.G., 1995, Simulation of an anatomically defined local circuit: The cone-horizontal cell network in cat retina, Vis. Neurosci., 12: 545-561.
Smith, R.G., and van Rossum, M.C.W., 1998, Noise removal at the rod synapse of mammalian retina, Vis. Neurosci., 15: 809-821.
Smith, R.G., and Vardi, N., 1995, Simulation of the AII amacrine cell of mammalian retina: Functional consequences of electrical coupling and regenerative membrane properties, Vis. Neurosci., 12: 851-860.
*Sterling, P. 1997, Retina, in The Synaptic Organization of the Brain, Fourth ed., (Gordon M. Shepherd, ed.), New York: Oxford Univ. Press.
Teeters J. L., and Arbib, M.A., 1991, A model of anuran retina relating interneurons to ganglion cell responses, Biol Cybern., 64:197-207.